One of our clients, Twila J Starkey, and I were chatting the other day about the many posts on this iron subject.
Her scintillating observation: “There’s just too much there! ”
And I agree.
This post is intended to Keep It Simple, Searchers (K.I.S.S.).
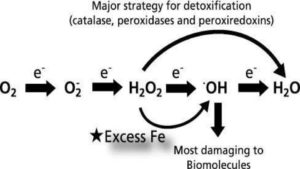
The pic above presents a compelling and straightforward diagram of what excess; unbound iron does inside our body, our organs, our tissue, and ultimately, our cells. So what happens is iron, when not properly bound by transport or regulatory proteins, is pro-oxidant, and thus, very toxic to our health.
Yes, I’ve said that before.
The pic below is intended to show just how ridiculously complex the process of oxidative stress can be. It comes from this epic penetrating article by Valko, et al. have gone out of their way to shed important light on the yin/yang of oxidative stress: “Can’t live with it/Can’t live without it.”

Fig.1. – Valko, M., et al. (2007). “Free radicals and antioxidants in normal physiological functions and human disease.”
https://www.sciencedirect.com/science/article/abs/pii/S1357272506002196
Some important questions for your to consider:
- Do you think your healthcare provider understands the chart above? I barely do and I have been studying this intensely for 3+ yrs!
- Can you see how high-dose iron supplements, or worse yet, iron infusions might bring those two diagrams to life, especially the second one?
- Can you see how measuring a single, controversial and metabolically inactive protein, ferritin, is not a proper, relevant, appropriate or complete measure to assess the complexity of iron metabolism?
What I would offer up as a key companion article to Valko, 2007 is an important study that I have shared before by Sir Douglas B. Kell, PhD entitled “Iron Behaving Badly”
Kell, D.B. (2009). “Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases.”
https://pubmed.ncbi.nlm.nih.gov/19133145/

Research Gate image
https://www.researchgate.net/figure/Some-effects-of-hepcidin-summarizing-the-fact-that-hypoxic-condition-can-suppress-it-and_fig8_23767901
I wonder how many have read the Douglas Kell article above? The difference between these two compelling studies is that Valko, et al., keep the illusion alive that oxidative stress can come from many metabolic sources.
Whereas Professor Kell puts the spotlight squarely where it belongs which is rogue iron found in the ferritin molecule. There was no pretense in his accomplishment of research he has done.
Know that there is 60x more iron than copper in the human body is very telling as stated by Gutteridge and Halliwell in their 1994 book.
Gutteridge, J.M.C., Halliwell, B. (1994). “Antioxidants in nutrition, health and disease.”
Then a bright member of the Magnesium Advocacy Group, Jim Urban, shared this expressive and penetrating piece on the toxicity of iron which I encourage you all to read:
Zacharski, L.R. (2014). “Ferrotxic Disease: The Next Great Public Health Challenge.”
https://academic.oup.com/clinchem/article/60/11/1362/5621712
If you really want to explore these iron issues and dynamics, here is the Full Monty Iron blood test that I routinely recommend to accompany the HTMA:
https://therootcauseprotocol.com/order-lab-tests/
The key is then getting it properly interpreted by someone who knows the difference between:
- Anemia of Chronic Inflammation which is quite common and caused by a state of excess iron that is in hiding in tissues, organs etc.
- Anemia of Iron Deficiency, which is next to impossible on a planet that has 36% of its composition, made up of iron.
I would like you to fully understand what Anemia of Functional Iron is really all about. Iron is supposed to be recycling not being stored. The understanding of iron status is so important especially if it is based on one marker, ferritin that is not telling the full story.
A votre sante!
MORLEY M. ROBBINS
For Facebook Discussion:
www.facebook.com/groups/MagnesiumAdvocacy/permalink/1126634560737945
