“Iron and the Red Blood Cell (RBC): Is this where the assault begins?” (OMg! Does a bear sleep in the woods?)
Dr Liz and I just watched the new movie on P.T. Barnum: “The Greatest Showman.” It’s definitely worth your time to witness the captivating story behind the indomitable spirit and imagination of this amazing figure in our entertainment past. The movie ended with one of the most famous quotes attributed to him:
“The noblest art is that of making others happy.”
I really like that sentiment, but I’m going to go one step further, and paraphrase it:
“The noblest act is teaching others how to be healthy.”
(I intend to adopt that statement as my signature effort in this lifetime)
I am devoting the rest of my days to helping people on this planet to get educated and to get empowered on the key steps that need to be undertaken to re-store metabolic balance in their bodies. It is not rocket science!
I should add that I am hardly alone in this effort, and among my many “mentors” that have helped shape and mold my thinking about these key requirements is Jerry Tennant, MD (Dallas, Texas), recently praised with a Lifetime Achievement Award at the Doctors Who Rock symposium in Florida. He is also the noted author of “Healing is Voltage,” among other books, which is a must read for anyone wanting to understand the wiring of the human metabolism, and the vital importance of cellular energy production to assure optimal cellular frequency therefore optimal health.
It’s worth knowing that what inspired Jerry Tennant, MD, at the depths of his 7-yr long illness crisis, was the fervent belief that if he could get one cell in his body to work, then he could get them all to work! (I should add, this focus is akin to the thinking of a gifted mechanic that I worked with, named Otis, one summer while on break from college. He was convinced that if he could get one cylinder in the engine to work, he could get them all to work.)
Given that focus, what then do I regard as the most important cell in the human body?
Well, I would argue that it’s the erythrocyte, as known as the red blood cell, or RBC. It’s been on my radar screen for ~8 years!
Okay, here are some key facts that highlight the importance of this ubiquitous and largely overlooked cell in our body:
- There are some 20-30 trillion RBCs in the average adult
- In one small drop of blood (1 cubic mm) there are 4-5 million RBCs
- RBCs account for ~84% of all human cells. (Please let that % sinks in before dashing to the next line.)
- RBCs, by virtue of being created inside the bone marrow, are considered connective tissue. (Again, please let the significance of that fact sink in!)
- Each human RBC contains ~250-270 million hemoglobin molecules
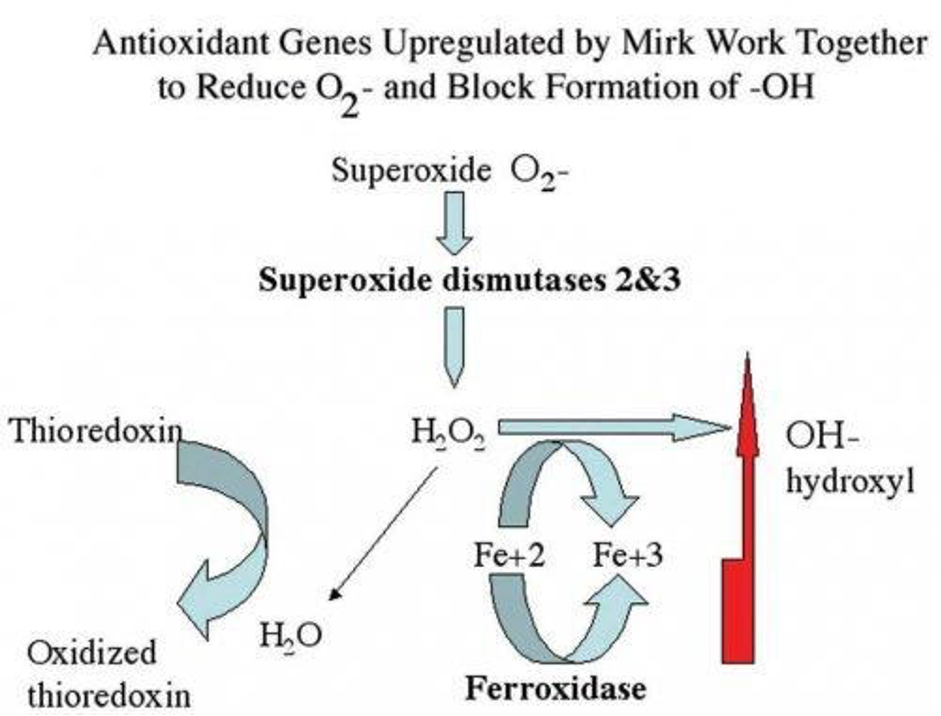
- There are 4 heme proteins contained in each molecule of hemoglobin, and each has an atom of iron in the center. (Not unlike a ceiling fan in the center of your den or living room)
- Each human red blood cell can carry 1 billion oxygen (O2) molecules (And if iron is misbehaving, that is a lot oxygen molecules to create potential rust, folks)
- 70 % of all iron contained in the human body is found in those zillions and zillions of haemoglobin
- The oft-quoted ideal lifespan for these RBCs is ~120 days
- The rate-limiting enzyme to determine the energy, viability and thus lifespan of these RBCs is G6PD enzyme, aka Glucose-6-Phosphate Dehydrogenase (It’s the head of the stream of the pentose phosphate pathway)
- “G6PD deficiency” is regarded at the greatest enzyme deficiency on this planet. It’s worth noting that this enzyme has relationships with both magnesium and bioavailable copper.
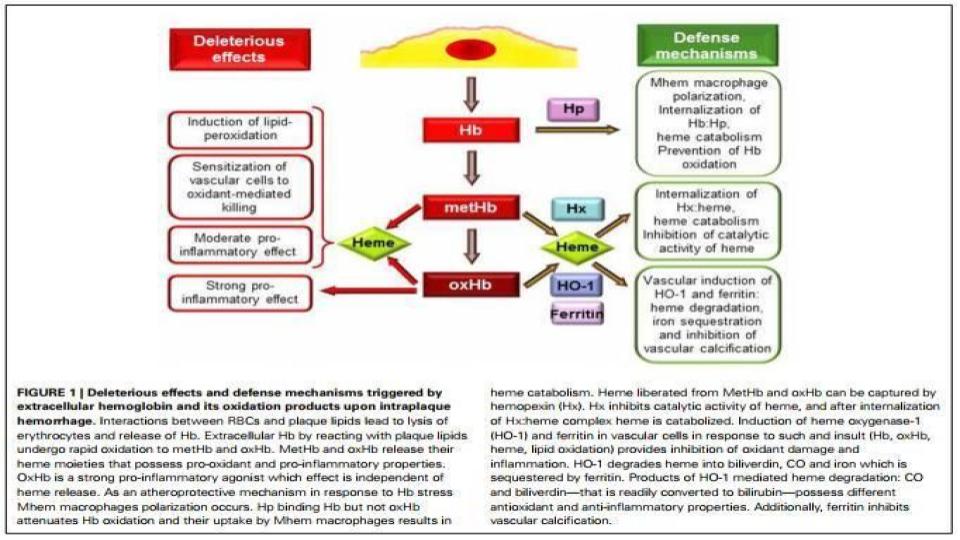
- When RBCs get stressed and can’t make proper energy, they lose their flexibility, and energy-driven shape, and then get “flagged” for re-cycling, as known as hemolysis.
- In separate studies, either copper deficiency or magnesium deficiency causes the RBC lifespan to drop 80% to ~20 days! That is an extraordinary loss of lifespan, and an extraordinary acceleration of RBC turnover!
- This turnover is the very metabolic mechanism that leads to iron-laden macrophages that results in all autoimmune conditions.
- It is worth repeating that the synthesis of heme, the insertion of iron into heme, the knitting of four heme to make one hemoglobin molecule, as well as the regulation of HO-1 (heme oxygenase-1) “re-cycling” enzyme all require bioavailable copper.
- Where does oxidative stress and inflammation start in our bodies? Right here in the RBCs (Red Blood Cells) of our blood.
- Most importantly, please remember that iron simply carries oxygen throughout the body, but for a fact, copper is the only mineral that can use or activate oxygen to release energy or change chemicals (e.g. neurotransmitters) in the body. That is a major big deal that is lost in the world of conventional “you need more iron” medicine.
Given all of this, doesn’t it make sense to start the process of understanding how to stop the oxidative chaos that originates inside the red blood cells?
Call me goofy, but this seems to me like a penetrating look into the obvious. Thankfully, I am not alone in this thought, as it has been a major focus of research for many decades. Regrettably, none of these findings about the importance and the fragility of the RBC seems to be taught at any school of health practitioner training anywhere on the planet in this modern era.
Here are some noted studies that offer keen insights to this iron <> RBC foundational premise:
Clemens, M.R., Waller, (1987). “Lipid peroxidation in erythrocytes”
www.ncbi.nlm.nih.gov/pubmed/3319229
“Most of the protective activity [for RBCs] is associated with two proteins, transferrin and ceruloplasmin.”
As has been noted before, iron requires constant supervision or chaperoning, not unlike a 4-yr old with a hammer!
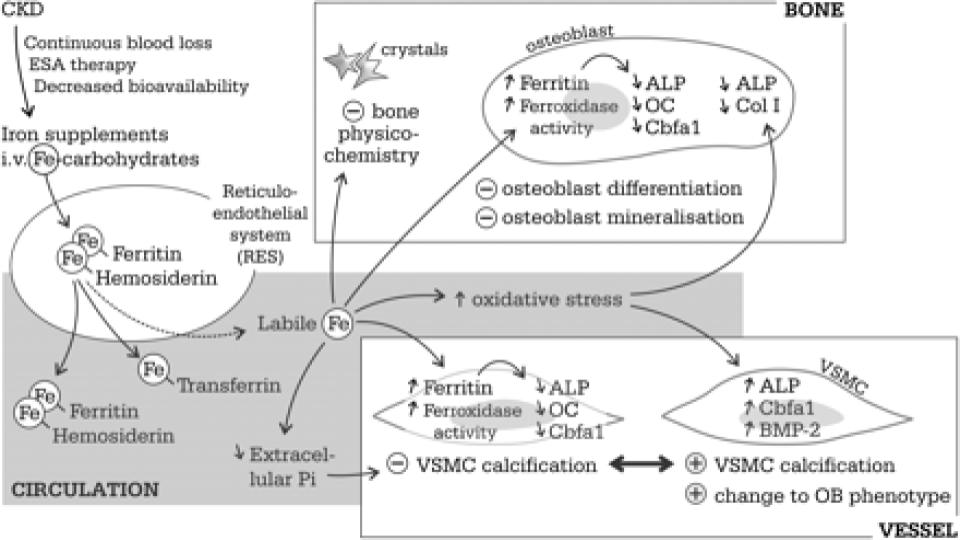
Neven, E., et al. (2011). “Iron and vascular calcification: Is there a link?”
academic.oup.com/ndt/article/26/4/1137/1884318
Figure 1 (below) of this seminal assessment of iron-induced calcification of patients with CKD is stunning. The fact that RBCs are considered connective tissue and have osteogenic [bone-making] properties is a game changer in terms of the recognized stimulatory role of excess, unbound iron in triggering and fostering plaque (atherosclerosis) and hardening of the arteries (arteriosclerosis), as well as calcification of organs throughout the body.