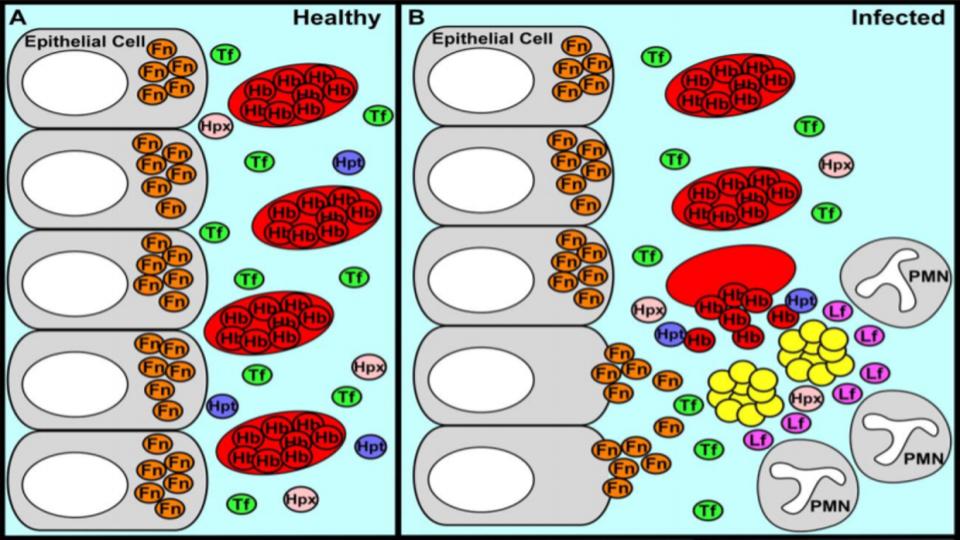
Why I Detest Hormone-D Supplements
- Hormone-D Supplements Deplete key minerals, especially Magnesium.
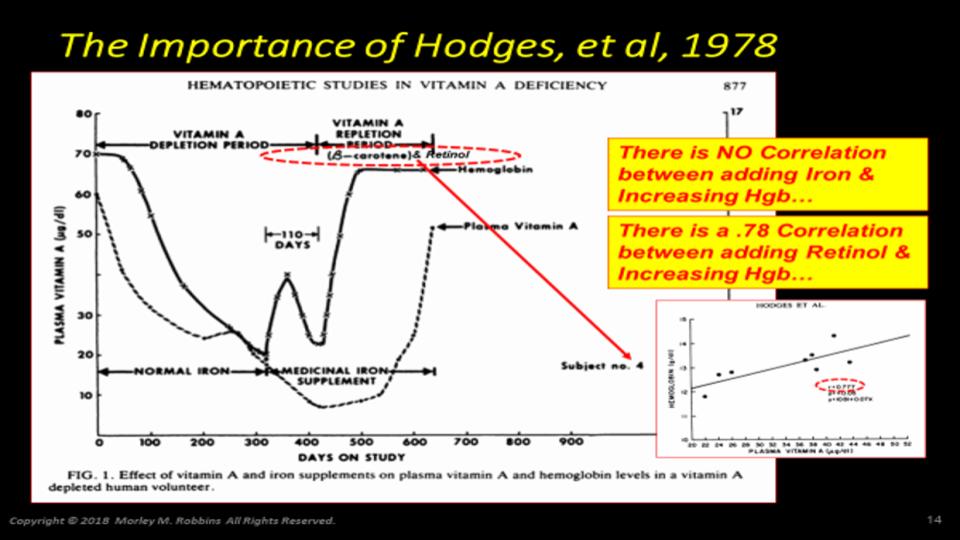
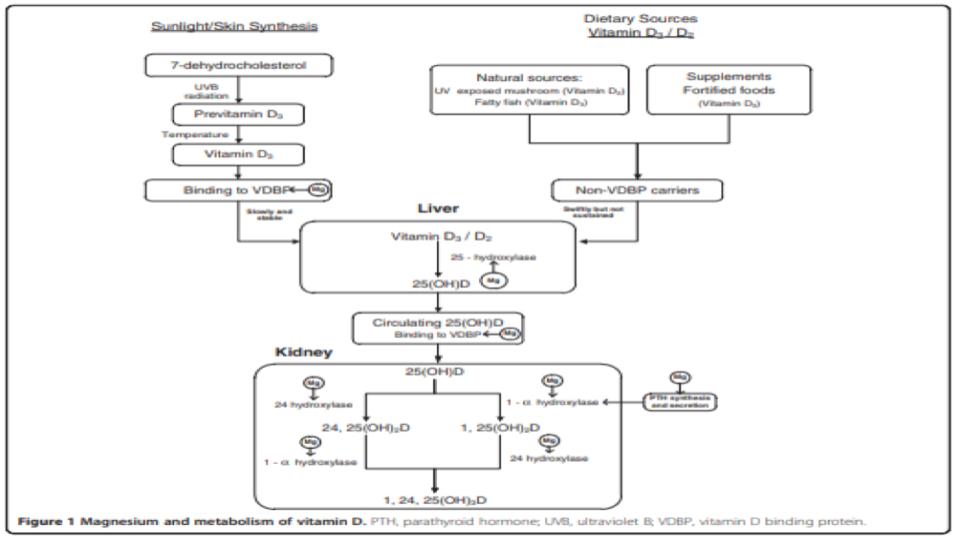
It is a well-established fact that Magnesium (Mg) is essential for many steps in hormone-D metabolism.
Please pay particular attention to Figure 1 at the top of this post and note all of the metabolic demands for Mg to support hormone-D transport and metabolic conversions in our liver and kidney.
Deng, X, et al. (2013). “Magnesium, vitamin D status and mortality: results from US National Health and Nutrition Exaination Survey (NHANES) 2001 to 2006 and NHANES III”
www.ncbi.nlm.nih.gov/pmc/articles/PMC3765911/
- Hormone-D Supplements are “D”eceptive about “correcting” Inflammation.
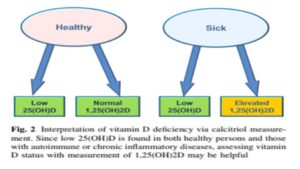
What often presents, as “low vitamin-D” on a routine blood test is merely a sign of inflammation. It is worth noting that inflammation is a clinical sign of Mg deficiency.
(Again, please see Deng et al 2013, linked above and also Weglicki & Phillips, 1992a).
What is wildly confusing to patients, and their practitioners, is that taking more hormone-D supplements does not, and will not, correct this inflammatory process, as is illustrated in Figure 2 (below):
Mangin, M., et al. (2014). “Inflammation and vitamin D: the infection connection”
www.ncbi.nlm.nih.gov/pmc/articles/PMC4160567/
- Hormone-D Supplements are diabolic in their impact on our Immune System.
- Taking vitamin-D, which increases the synthesis of 1,25(OH)2D3 (active hormone-D), makes cells more sensitive to hydrogen peroxide (H2O2). It is worth noting that all inflammatory cytokines stimulate the increased production of H2O2, which is a known by-product of inflammation.
Krane, S.M., et al. (1990). “1,25-Dihydroxyvitamin D3 increases the toxicity of hydrogen peroxide: the role of calcium and heat shock.”
www.ncbi.nlm.nih.gov/m/pubmed/2226658/
- Taking vitamin-D dulls the immune system.
Contrary to popular opinion, there is no “Causal Connection” between vitamin-D intake and improvement in either innate or adaptive immune function. An important finding from a recent study presents a clearer picture of this little discussed reality:
“Although, unlike Vitamin-D deficiency and rickets, a causal link between vitamin D deficiency and immune disorders has not yet been proven, there is compelling evidence in the laboratory of beneficial effects of 1,25(OH)2D3 in inflammatory diseases where Th1 cytokines have a pathological role.”
Please know that there is far more to this story than the simple-minded emphasis on ingesting more hormone-D!
Wei R., Christakos, s. (2015). “Mechanism underlying the regulation of innate and adaptive immunity by vitamin D.”
www.ncbi.nlm.nih.gov/pmc/articles/PMC4632412/
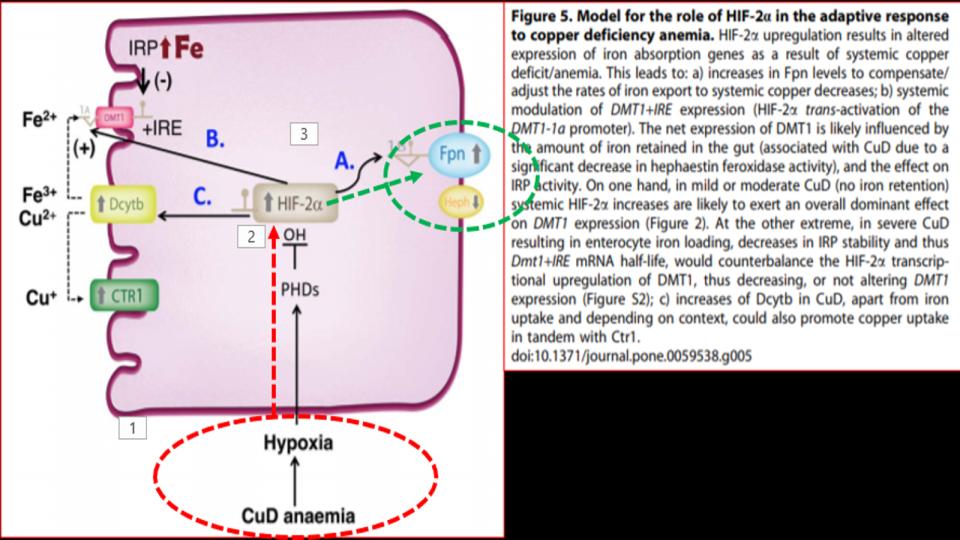
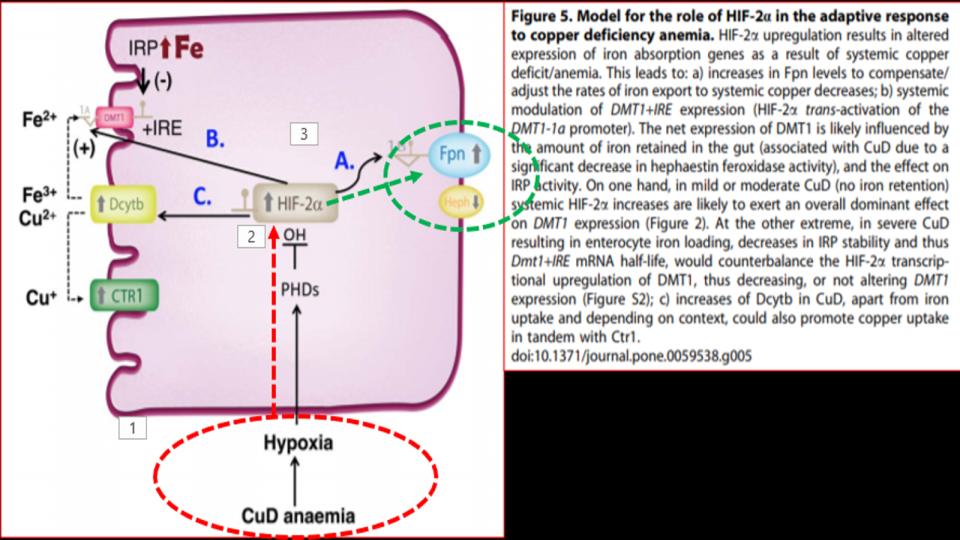
- Hormone-D Supplements are demonic by altering key genes involved in Iron metabolism.
a) Contrary to popular belief in “high” vs. “low” thinking re nutritional metabolites, the body actually works off of optimal levels. Truth be known, our bodies run on a bell-shaped curve, which I prefer to call the “Goldilocks Syndrome.” (Far better to be at the peak, than at either pole!)
Given that we’re now learning that vitamin D Is a regulator of Endothelial Nitric Oxide Synthase (eNOS), might the unchecked intake of this hormone be another important factor to consider in our metabolism getting out of balance and our genes getting *tweaked*
Andrukhova O., et al. (2014). “Vitamin D Is a Regulator of Endothelial Nitric Oxide Synthase and Arterial Stiffness in Mice”
www.ncbi.nlm.nih.gov/pmc/articles/PMC5426652/
b) It’s worth noting that 20 years before learning about the impact of hormone-D supplements on eNOS, it was discovered that N.O. (Nitric Oxide) has a key regulatory role over IRPs (Iron Regulatory Proteins).
IRPs are an essential metabolite in directing iron metabolism function through their direct control of ferritin mRNA translation, as well as Transferrin Receptor-1 mRNA stability. This relationship of IRP<>IRE (Iron Responsive Elements) is among the most important, and sadly, among the most confusing aspects of iron metabolism.
Suffice it to say that in the absence of N.O., these IRPs act very differently, and that in the increased presence and prevalence of N.O., both the storage and absorption of iron, as well as the synthesis of hemoglobin are affected.
The net effect: Increased N.O. creates the illusion of iron deficiency by virtue of its impact to decrease the synthesis of ferritin, and increase the synthesis of transferrin receptors to absorb more iron.
That series of changes is a recipe for iron overload that presents as low ferritin, that wrong perception of low iron.
Pantopoulos, K. Hentze, M.W. (1995). “Nitric oxide signalling to iron-regulatory protein: Direct control of ferritin mRNA translation and transferrin receptor mRNA stability in transfected fibroblasts”
www.pnas.org/content/pnas/92/5/1267.full.pdf
Pantopoulos, K, Weiss, G., Hentze, M.W. (1994). “Nitric oxide and the post-transcriptional control of cellular iron traffic.”
www.sciencedirect.com/science/article/abs/pii/0962892494901791
c) And in a stunning study on this same metabolite, we learn that N.O. has an effect on 3 key genes — Post-Translationally — that are central to iron metabolism. These finding lend greater specificity to the findings of Pantopoulos & Hentze noted previously:
- Decreased y-Globulin/eALAS function that affects Hgb synthesis;
- Decreased H-Ferritin synthesis that affects storage of intracellular iron;
- Increased TfR1 (Transferrin Receptor-1) that increases iron absorption
Domachowske, J.B. et al. (1996). “Nitric Oxide Alters the Expression of y-Globin, H-Ferritin, and Transferrin Receptor in Human K562 Cells at the Posttranscriptional Level”
www.bloodjournal.org/content/bloodjournal/88/8/2980.full.pdf?sso-checked=true
d) Finally, presented in this section are two key quotations from noted iron researchers, Guenther Weiss, PhD, William Hentze, PhD and colleagues, that serves to punctuate this profound physiological mechanism by N.O. that is little discussed, nor apparently properly understood in clinical circles.
A loose translation of this set of findings (#4a-#4d ^^^^) is that repeated supplementation with hormone-D appears to contribute to the oft declared diagnosis of “anemia,” that is overtaking the metabolic landscapes of patients worldwide.
It turns out that “low ferritin” and “low hemoglobin” may have a completely different origin than the habitually assumed declaration of low iron:
“We conclude from these data that N.O. controls ferritin biosynthesis by changing the rate of translation of its mRNA”
“We have established that N.O. synthesized by macrophages and non-macrophages regulates IRF [today, known as “IRP”], the central Post-Translational Regulatory molecule of mRNAs involved in iron metabolism”
Weiss, G., Hentze, W.M. et al. (1993). “Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway.”
www.ncbi.nlm.nih.gov/pmc/articles/PMC413641/
5. Hormone-D Supplements are destructive to Optimal Metabolism
a) In a study published over 55 years ago, it was revealed that vitamin-D supplements cause renal potassium wasting. Please know that depleting our cells of potassium, which is where potassium hangs out in our bodies, makes our cells increasingly vulnerable to an increase in intracellular iron that comes by way of high dietary iron and increased Transferrin Receptor activity as noted in the sections above.
Again, this is not a desirable mineral imbalance (i.e. low K vs. high Fe) in the short-term, and certainly not in the long-term!
Ferris T.F. et al. (1962). “Renal potassium–wasting induced by vitamin D” www.ncbi.nlm.nih.gov/pmc/articles/PMC291035/
b) Vitamin-D supplements cause ATP depletion and iron-mediated attack. Again, this appears to be a little-known effect of hormone-D supplements, but the impact to optimal metabolic function is stunning, especially in our kidney tissue.
Zager, R.A.,1999, “Calcitriol Directly Sensitizes Renal Tubular Cells to ATP-Depletion- and Iron-Mediated Attack”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1866639/
c) Vitamin-D destroys vitamin-A reserves.
Mawson, A.R. (2012). “Role of Fat-Soluble Vitamins A and D in the Pathogenesis of Influenza: A New Perspective”
www.hindawi.com/journals/isrn/2013/246737/
A key footnote [#96] in Mawson (2012) examines a never-discussed dynamic of vitamin-D levels affecting vitamin-A status and stores in the liver and the body. And despite what the title says on this study (Aburto et al., 1998), here is a key sentence from Mawson (2012) that is drawn from this critically important footnote:
“Although little is known about the effect of reduced sunlight exposure and/or deficient vitamin D levels on vitamin A metabolism, even small to moderate doses of vitamin D in chickens reduce liver vitamin A stores and lower the level of vitamin A in blood” [96].
I was stunned the first time that I read that sentence re the impact of vitamin-D on vitamin-A!
Does anyone honestly think that high intakes of this hormonal, supplemental-D would act any differently inside our (chicken-fed) bodies? Is anyone else just a little suspicious that vitamin-D is always described as being “deficient,” and vitamin-A is always found to be “toxic” in the literature?
Hmm?
Please know, this is the only study (Aburto et al, 1998) that even hints at this impact of hormone-D on vitamin-A. Know that I have been looking for validation of this fact for a number of years.
Aburto A, Edwards HM Jr., & Britton WM, (1998). “The influence of vitamin a on the utilization and amelioration of toxicity of cholecalciferol, 25-hydroxycholecalciferol, and 1,25 dihydroxycholecalciferol in young broiler chickens,”
https://www.sciencedirect.com/science/article/pii/S0032579119408523
6. Hormone-D Supplements deflect the truth about what sunshine does for us.
a) Pine trees, grown in acidic (high iron) soil, produce copper rich products especially vitamin-C.
www.wildedible.com/pine-needle-tea-natural-vitamin-c
There are similar properties with citrus fruit trees, oak trees with acorns, pecan trees with pecans, etc. Wouldn’t it be valid to say that vitamin-C, too, is a valid candidate to be the “sunshine” vitamin, given the role that sunshine plays in this biological transmutation?
b) Sunlight activates POMC, which is a key catalyst to the PAM enzyme of the Hypothalamus that regulates 12 other key enzymes/hormones in our body. Again, it’s fascinating that this powerhouse of transactions is triggered by sunlight >> POMC:
“Sunshine activates a gene called POMC, which in turn helps create melanin that determines skin color. This beneficial gene enhances sex drive (the endorphins or ‘happiness hormones’), as well as leptin, which helps burn fat and keep you thin.”
Skin Biology, Pickart L. (2019) “Sun Health”
https://therootcauseprotocol.com/wp-content/uploads/2024/05/Sunlight-and-Health.pdf
c) Sunlight activates the catalytic breakdown of retinol that is key to its impact.
Given the worldwide obsession with storage-D status, it is both surprising and important to learn of the interdisciplinary and interactive nature of vitamins A<>D:
“For instance, solar radiation has opposite effects on vitamins A and D, catabolizing vitamin A but increasing the concentration of vitamin D; the effects of the two vitamins are mutually inhibitory; retinoids regulate airway epithelial cell growth, differentiation, and gene expression…”
And when you fully understand the myriad metabolic roles of vitamin A, and how it is the downstream metabolites from Retinol and Retinoids that have such profound effects on our metabolism, suddenly the role of sunlight in our lives (and livers!) takes on a much deeper and more important role than previously thought.
And what ubiquitous agent blocks these key retinol-related events? None other than “Sunscreen!” Please know, our skin “burns,” not from a lack of sunscreen, but a lack of fat-derived, Retinol.
Mawson, A.R. (2012). “Role of Fat-Soluble Vitamins A and D in the Pathogenesis of Influenza: A New Perspective” (Link listed above in 5c)
d) Then we learn that sunlight converts a key Cytochrome P-450 enzyme, eNOS, to enable the transformation of fat soluble vitamin-D <> vitamin D3 Sulfate, a water-soluble (i.e. usable) form of vitamin-D.
As I understand this process, it is only sunlight that can activate this critical enzyme mechanism. And the importance of this transformation is equally profound for our optimal health, as thoroughly explained in this series of interviews between Stephanie Seneff, PhD and Joseph Mercola, DO [No longer available]:
Regrettably, what is absent in this ^^^^ otherwise powerful and informative exchange between these two “greats,” is any consideration of the importance or the interaction with real vitamin-A, aka Retinol in this process. And why is this so important to our health
Because retinol, and its derivatives, are considered the “universal chromophore”, they have unique properties to harvest and harness light! There is a powerful connection between our eyes, and our liver, both organs being dependent on real vitamin-A, aka retinol.
Do we now have yet a third potential vitamin candidate to be the “sunshine” vitamin?
It is curious how the very start of the process to make vitamin-D depends on sunlight to activate a Cytochrome P-450 enzyme that requires copper, and that the critical VDR (Vitamin-D Receptor) is helpless without the direct involvement of a key Retinoic Acid Receptor, RXR derived from the metabolic breakdown of Retinol.
Musings of a mineral geek –
Zhong, M et al. (2012). “Retina, Retinol, Retinal and the Natural History of Vitamin A as a Light Sensor”
www.ncbi.nlm.nih.gov/pmc/articles/PMC3546623/#!po=0.515464
7. Hormone-D dulls our potential for Optimal Health
In what is regarded as the most robust Meta-Analysis of vitamin-D across a wide variety of conditions, a key summary comment from this analysis of some 187 other studies seems to identify the emerging and uncomfortable truth re the hormone-D hysteria gripping the planet:
“Recent reports have highlighted the lack of concordance between observational studies and randomized controlled trials, concluding that vitamin D is more likely to be a correlate marker of overall health and not causally involved in disease.”
When we were kids, the expression was “all bark, and no bite!”
Trust me, the dreary bloom is falling off this dietary daffodil!
Theodoratou E, Ionnidis JPA et al. (2014). “Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials”
www.bmj.com/content/bmj/348/bmj.g2035.full.pdf
And just to solidify your understanding of the restrained “need” for vitamin-D, there is no clinical benefit to having storage-D (25(OH)D) >21ng/dL. This was the sterling and unsettling conclusion of a recent study to contrast vitamin-D status against all-cause mortality.
This figure flies in the face of the absurd Lab ranges for storage-D that start at 30 and go up to 100! The 2014 Study noted ^^^^ above is a chilling indication of how out of touch the meme is about hormone-D, which is amplified in Dr. Amer’s equally deflating conclusions below.
Amer, M. et al. (2013). “Relationship between 25-Hydroxyvitamin D and All-cause and Cardiovascular Disease Mortality”
www.amjmed.com/article/S0002-9343(13)00084-3/pdf
Ok, that is a lot of info. Please forgive the download.
I realize that this post is a doozy, but I’ve been collecting these articles for months, and decided it was time to do the definitive Vitamin-Dump!
Please know, I will be drawing upon several of these studies for posts in the next several weeks/months as I plan to do a series of Posts to drill deeper into the 2nd and 3rd order implications of these dynamics.
So, let’s drop the pretense. There is a great deal more to this synthetic, soy-based (not sun-based) toxic supplement than you are being led to believe on the Internet and in your doctor’s office.
It is not a panacea supplement for improving health.
In fact, following my close to 10 years of analysing and evaluating this issue, I would argue that the use of hormone-D supplements represents a central pathway to weaken our mineral dynamics, our metabolic pathways, and ultimately, our immune system.
And finally, an important caution, please beware of studies that are based on Correlation, and not Causation. A critical question to ask yourself, and to amplify this point: “Do flies cause garbage?”
Of course not! And this is true of hormone-D, too!
It appears that “Low vitamin-D” shows up (i.e. it is correlated) with countless conditions, but its lack is hardly a cause of these many conditions, and its supplementation is hardly a correction of these same conditions. (Please take a moment to re-read that sentence, once again) Worse yet, it seems that the much bally-hooed “benefits” of taking this synthetic supplement are more illusory, than real. Why are we not surprised?
In conclusion, supplementing with this “poison,” because you think you have a dearth of this storage hormone in your blood, could very well be the stimulus for the death of your vitality, in ways you never imagined possible.
“A” votre sante!
Morley M. Robbins