This post is about Eye/Iron or Eye-ronic origin of Alzheimer ’s disease and all other forms of neurodegeneration known on this Planet.
“Discovery consists in seeing what everybody else has seen and thinking what nobody has thought.”— Albert Szent-Gyorgyi, 1937 Nobel Laureate: “Discovery of Vitamin ‘C’ complex”
I have wanted to write this post since the beginning of 2017, but several key pieces of the puzzle were cleverly eluding me. Please know that I am far from done understanding all of these dynamics of neurodegeneration, but I am reasonably confident now to make several key assertions, which I’ll do throughout this post with a wrap-up at the end.
I am quite confident; many of you will not entirely like what this post has to say. You will be challenged to understand the technical aspects of it and I barely have a full grasp. But the mineral and metabolic issues that are covered in this post will grow in importance, and will become a key cornerstone in our understanding of how best to get out of our iron shackles and regain our metabolic homeostasis.
Some notable facts about neurological conditions, in general, and neurodegenerative issues, in particular:
- One billion people, worldwide, nearly 1 in 6, suffer from neurological disorders: AD, PD, MS, strokes, epilepsy, migraines, brain injuries and other forms of neuroinfection.
- Thirty seven million people worldwide suffer with Alzheimer’s
- Ten million people worldwide suffer with Parkinson’s
We all know at least 1-2 people (family members, friends, work associates, neighbors, etc.) that are being ravaged by the information that surrounds these brain-related neurodegenerative conditions. It is absolutely time for this level of clinical inadequacy to stop.
I’m doing my part today by sharing this extensive panel of research to compile the leading thinking in a way that presents what I regard as a compelling argument about a major destructive factor that is right under our noses. Which is contributing to the unchecked acceleration of neurodegeneration and misery on this planet.
So what’s really going on here with these neurodegenerative diseases? Isn’t that what you’ve come to expect to learn innovative and foundational metabolic nuggets from these “Insightful Teaching Posts” (otherwise known as Iron Toxicity Posts)?
- It’s important to know that Alzheimer’s disease (AD) is characterized by the following mineral patterns in the brain tissue:
– High copper,
– Low iron,
– Low zinc, typically
– Low ferroxidase function (ceruloplasmin),
– High oxidized serotonin, especially in the hippocampus
– Confusing reference(s) to Monoamine Oxidase (MAO, as a bad guy) that is meant to “turn off” neurotransmitters
– Silence on melanin’s role in the locus coeruleus that affects the hippocampus
- It’s equally important to know that Parkinson’s disease (PD), on the other hand, is characterized by these mineral patterns in the brain tissue:
– Low Copper,
– High iron,
– Low zinc, typically…
– Low ferroxidase function, once again,
– High oxidized dopamine, especially in the substantia nigra
– Confusing reference(s) to MAO (as a “bad guy”), again, that is “turning off” the neurotransmitters
– More silence on melanin’s role in the locus coeruleus that affects the substantia nigra.
Clearly, there are some notable and contrasting mineral dynamics, which adds to the mystery and clinical confusion about these two most prevalent forms of neurodegeneration on the planet.
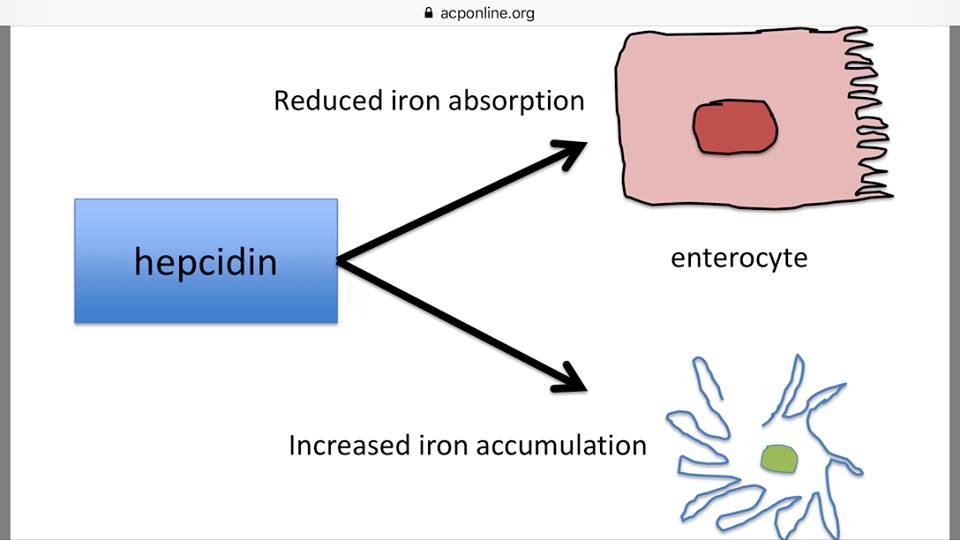
But please understand the fact that both conditions, and all neurodegenerative conditions, collectively, that I’ve studied, have a notable lack of ferroxidase function.
This is a critical point to which we will return shortly.
Okay, so with that as a background, let’s begin this blinding blitzkrieg blog!
I’ve known for quite some time that EMFs, you know those pesky little electromagnetic frequencies that every conceivable device we use emits has an effect on our iron status. In effect, this is a ubiquitous form of environmental stress. What we’ve learned is that there is always a Magnesium Burn Rate (MBR) attached to any form of stress.
EMFs are no different in their Magnesium (Mg) depleting effects, and if Mg goes down, trust me, the toxicity of iron is going to be going up! Here are several blockbuster articles for those of you seeking to better understand this EMF dynamic to get a better handle on how this tidal wave of radio frequencies is altering not only our external environment, but also our internal antioxidant enzyme landscape:
Maaroufi, K., et al. (2014). “Spatial learning, monoamines and oxidative stress in rats exposed to 900 MHz electromagnetic field in combination with iron overload”
pdfs.semanticscholar.org/29a3/7abf4734218e0e6276afd52ff04c59650562.pdf?_ga=2.68949969.1975053235.1498943746-1468208896.1498943746
Gherardini, L., et al. (2013). “Searching for the Perfect Wave: The Effect of Radiofrequency Electromagnetic Fields on Cells” (The impact on Complex IV, cytochrome c oxidase is particularly noteworthy)
www.ncbi.nlm.nih.gov/pmc/articles/PMC4013569/
Consales, C., et al. (2012). “Electromagnetic Fields, Oxidative Stress, and Neurodegeneration”
www.hindawi.com/journals/ijcb/2012/683897/citations/
Please note that the first study noted (Maaroufi, 2014) is a very provocative one that chose to combine EMFs (900 MHz) with iron loading on the experimental rodents. The sentence that really caught my “eye,” (pardon the pun) was the following:
“A link between EMF, iron accumulation in the brain and neurodegenerative disorders including Parkinson’s and Alzheimer’s diseases has been suggested.”
Are you sitting up a bit straighter in your chair right about now? So, I’ve known about these iron properties of EMFs for quite a while, and I knew that there had to be a connection to the use of blue light that many of these devices emit, but I lacked the causal connection between that high-energy spectrum of light and its impact on iron. There is an absolute relationship between copper and the copper-dependent enzymes [think cytochrome c oxidase] and the other low energy end of the light spectrum where red and infrared light hangs out.
Everything changed a couple of weeks ago when my friend and colleague Alicia Barone Stephens happened to post an article about “Blue Light” and “Alzheimer’s” that had some critical commentary & insights from brain surgeon, Jack Kruse, MD.
“Culprit in Plain sight in Alzheimer’s disease development”
medicalxpress.com/news/2017-06-culprit-hidden-plain-sight-alzheimer.html
Jack Kruse, MD commenting on Facebook:
www.facebook.com/drjackkruse/posts/1798230000241346
And I quote from Jack Kruse, MD:
“Too bad no one in this paper realize that transition metals, like Iron, draw nnEMF [non-native EMF] toward them because of the D shell electrons they contain. That is why AD is blowing up in a blue-lit microwaved world. CULPRIT HIDDEN IN PLAIN SIGHT IN ALZHEIMER DISEASE DEVELOPMENT”
It doesn’t get too much clearer than that. I’m delighted that Jack Kruse, MD elected to connect these dots so precisely. What is vital to understand is that our eyes are rich sources of iron to support the high metabolic demands of our eyes, and yet this pesky heavy metal accumulates as we age. This is not my conjecture; it is addressed in almost every article that I’ve read about eye disorders and disease over the last two years.
Here’s one of ~35 that I’ve read on this eye/iron dynamic:
Loh A. et al. (2009). “Iron homeostasis and eye disease”
www.ncbi.nlm.nih.gov/pmc/articles/PMC2718721/pdf/nihms-125393.pdf
Okay, so what do we know thus far?
- The incidence of neurodegeneration is exploding all over this planet.
- We know that the two most prevalent forms, AD and PD, have notable mineral differences, yet notable overlaps in key enzyme dynamics that cause these so-called conditions.
- We know that our contemporary and constantly connected environment is teeming with technology that is causing both oxidative stress, and has known connections to the very heavy metal that fuels the relentless oxidative stress that ails us.
- And while we’ve talked about this iron-induced oxidative stress many times before, the spin has always been around our food and dietary intake of enriched iron not our eyes!
What I want to emphasize in this iron toxicity post #61, is that there are added nuances to this crisis that even I was unaware of until forcing myself to sit down and piece this puzzle together. And I’m hardly done, despite all the hours, and hours of effort to read, synthesize and now write this unsettling piece.
As it turns out, our daily intake of our popular and dependent technology is only adding to our growing metabolic woes, especially as we age. Trust me, I would much rather be outside enjoying this beautiful Saturday (July 1, 2017) than be zapping my eyes, my thyroid (thank you Jack Kruse for that keen nuance that I missed!), as well as my brain, with this nnEMF/Blue Light emitting, oxidative stress-creating computer screen! But I figure you all are worth it!
Alright, if there is anyone at this point that doubts that the foundational flaw in the conventional understanding of the meteoric rise in the prevalence of AD or PD has nothing to do with a lack of ferroxidase enzyme function to keep oxidative stress in the brain at bay, please note the following studies:
A sampling of proof that Alzheimer’s and Parkinson’s are connected to copper<>iron dysregulation:
Kristinsson, J., et al. (2012). “Ceruloplasmin and iron in Alzheimer’s disease and Parkinson’s disease: a synopsis of recent studies”
www.ncbi.nlm.nih.gov/pmc/articles/PMC3493298/
Torsdottir, G. et al. (2011). “Ceruloplasmin and Iron Proteins in the Serum of Patients with Alzheimer’s Disease”
www.ncbi.nlm.nih.gov/pmc/articles/PMC3243634/
Montes, S., et al. (2014). “Copper and Copper Proteins in Parkinson’s Disease”
www.hindawi.com/journals/omcl/2014/147251/
These are merely three of ~50+ articles that I have read and analysed to get to the root cause of this maddening and frightening neuro-crisis. Up to this point, I was quite clear and confident that the liver was the single greatest producer of ferroxidase enzyme (FOX, or ceruloplasmin oxidase, or Cp), and that the brain was #2. That hierarchy had been noted in dozens of recognized and repeatedly referenced articles. It is worth noting that FOX is also made in the endocrine glands, the kidney, the uterus, the genitals, the breasts, etc., anywhere there is high metabolic activity to address the potential toxicity of iron found in oxygen-rich haemoglobin.
Everything changed when I read this mind-numbing study by Lin Chen, and colleagues at the University of Pennsylvania, at the start of this year:
Chen, L., et al. (2003). “Increased Expression of Ceruloplasmin in the Retina following Photic Injury”
Abstract: iovs.arvojournals.org/article.aspx?articleid=2416930
Full Article: www.molvis.org/molvis/v9/a22/v9a22-chen.pdf
Please take a moment to familiarize yourself with these findings and let the significance of this study sink in, in light of all that we’ve covered, especially these past two years. What Dr. Chen and colleagues discovered is that the eyes make 8 x more ferroxidase enzyme (ceruloplasmin) than the brain. Do you understand the magnitude of these findings? That it is our eyes that are not only taking it “on the chin” with the increased oxidative stress, but that they are supposed to be making eight times more ferroxidase than our brains in a nutritional/medical environment that has gone out of its way in the past 50+ years to destroy the body’s and the eye’s ability to make ferroxidase enzyme. Then on top of that, add insult to injury by going on to not even bother to measure it. (Thus, the infamous “not known, and not looked for” medical mantra)
Do you finally understand why I despise hormone-D so much? That obnoxious, synthetic, toxic supplemental hormone that is completely misunderstood by scientists, clinicians and the masses kills retinol, the very metabolic backbone for making this vital ferroxidase enzyme.
Isn’t it interesting that we are chided for using retinol, even though it is essential for the very function and metabolic activity of the retina? Get it? Retinol for the retina! Do you now finally understand my obsession with the Root Cause Protocol, is inspired by Mother Nature, is designed to enhance the viability and function of the ferroxidase enzyme (FOX, ceruloplasmin oxidase, and Cp)?
In case you think that that retina<>ceruloplasmin was a “one-and-done-study,” here’s what that provocative team based at UPenn went onto discover, in addition to their blockbuster revelations about FOX:
Hahn, P., et al. (2004). “Disruption of Ceruloplasmin and Hephaestin in mice causes retinal iron overload and retinal degeneration with features of age-related macular degeneration”
www.ncbi.nlm.nih.gov/pmc/articles/PMC518844/pdf/10113850.pdf
So, here we have proof that the very disruption of ferroxidase function, as expressed in both ceruloplasmin and hephaestin (an enzyme cousin of Cp), causes iron to build, which then goes on to create even more oxidative stress.
So, has anyone been wondering why I chose the graphics for this particular Post?
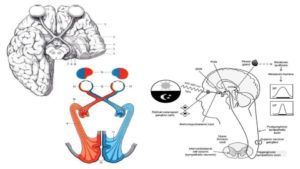
What we are seeing in those pictures is the unique structure and placement of the eye, it’s optical nerve and the amazing optic chiasm [Greek for “criss-cross”] that creates an “X” in a particularly important and vulnerable part of the brain:
- The pituitary & thyroid are below that chiasm.
- The limbic region of the brain (think master regulatory regions, like the hypothalamus, etc.) is directly above that chiasm.
Do you think that there just might be building, wandering, and destructive iron-induced oxidative stress that is associated with the retina(s), and subsequently the optic nerve(s), that is compromised in its ability to make the very antioxidant enzyme that must be vitally important for our optimal health? Or why would our maker have enabled us to make so much of this antioxidant enzyme, and in that particularly sensitive area of our body?
It would be reasonable to think that the systemic and progressive lack of this ferroxidase thingy is the sole reason for all this chaos, but as Mark Twain might say: “It just AIN’T so!” There are other antioxidants involved, as well. There are brain chemicals known as prion proteins, and it was just today that I learned that they are copper dependent. I had not learned that in my prior readings. These key proteins play very important roles as an antioxidant, but they get functionally tweaked, especially when they’re in the presence of iron-laden macrophages, that are called microglia and astroglia, which are found in the brain region.
Here are some compelling studies that support the role of prion proteins, but also how they are being accused, wrongly, in my humble opinion, for being a source of the problem, when they are seeking to come to the aid of the oxidizing tissue. Nothing like blaming a key component that is being affected by iron-toxic macrophages that alter the optimal function and physiology of these copper-driven chemicals.
Price, D.L., et al. (1993). “Alzheimer’s Amyloid Plaque and Prion Diseases”
www.ncbi.nlm.nih.gov/pmc/articles/PMC46935/pdf/pnas01471-0012.pdf
Kercher, L., et al. (2007). “Prion Protein Expression Differences in Microglia and Astroglia [Macrophages] Influence Scrapie-Induced Neurodegeneration in the Retina and Brain of Transgenic Mice”
jvi.asm.org/content/81/19/10340.full.pdf+html
Zhang, W., et al. (2014). “Role and mechanism of microglial activation in iron-induced selective and progressive dopaminergic neurodegeneration”
www.ncbi.nlm.nih.gov/pubmed/24277523
It does not stop there, unfortunately. Anyone who has loved ones suffering from these neurological conditions, especially Alzheimer’s, is aware of amyloid protein precursor (APP) that, as it turns out, actually has ferroxidase enzyme function. APP is seeking to quell the rising oxidative stress that is coming from the daily intake of iron-enriched food and our never far-from-us iron enriching electronics!
Duce, J.A., et al. (2010). “An iron-export ferroxidase activity of β-amyloid protein precursor is inhibited by Zinc in Alzheimer’s Disease”
www.sciencedirect.com/science/article/pii/S0092867410009384
www.ncbi.nlm.nih.gov/pmc/articles/PMC2943017/
Take stock of the added significance of this study by noting that zinc inhibits the ferroxidase activity of this critical brain tissue antioxidant. This is consistent with other studies that have proven that zinc zaps ferroxidase function, which we have examined in prior posts. Please, do not use zinc supplements, under any circumstances, if your ultimate objective is to increase the level and functionality of your ferroxidase enzyme. You can get zinc from your diet, not from a bottle.
Let’s keep it simple, seekers. Let me quote the last sentence of the abstract of this illuminating study by Duce, 2010:
“Iron causes selective and progressive dopaminergic neurodegeneration, and microglial NOX2 activation potentiates the neurotoxicity. PKC-σ, P38, ERK1/2, JNK, and NF-КBP65 are the potential molecules relevant to microglial NOX2 activation.”
It would be wonderful if more practitioners, pundits and FB posters would be this direct about all this clinical chaos occurring inside the brains of their patients and readers!
The three most dangerous minerals, in a supplemental form, that are the mainstays of allopathic practitioners are calcium, iron and zinc. They are not the answer, they have never been and they never will be. I only hope that more readers will have fresh eyes to see the mineral truths in this post #61.
Foundational iron hypothesis:
“Iron and iron dysregulation, due to a systemic lack of bioavailable copper, causes neurodegeneration period!”
What is rarely, if ever addressed, are the many key brain and neural tissue antioxidants that are being under produced and/or compromised time, and time again, throughout the brainstem and the brain cortex tissue.
We come to learn that it starts in our eyes! The bulk of conventional neurodegeneration research blows entirely past the central role of ferroxidase enzyme, prion proteins as key antioxidants, APP as yet another key antioxidant enzyme. What I am now discovering is that there is a granddaddy anti-oxidant of them all, it’s called melanin.
Melanin (pigmentation) is found in hair and skin and guess what, it is found in the eyes too. It is not about the colour of the eye and no, I am not talking about melatonin, I am zeroing on melanin. Melanin has a key chemical that plays a central role in many areas of the brain, not the least of which is the locus coeruleus [which means black dot], which is a major source of making dopamine to influence the functionality and balance of many, many sections of the brain.
Arias-Esparza, M., et al. (2011). “The Unexpected Capability of Melanin to Split the Water Molecule and the Alzheimer’s Disease”
https://www.scirp.org/journal/PaperInformation.aspx?PaperID=7404
This study above study is an absolute must read. This study breaks critical new ground, and shatters the very foundation of our known knowledge of cellular energy, electrical transmission around the body, as well as key anti-rusting factors, to name but a few functions, that have been shielded from us. I know not why, but I have a few theories.
No, melanin is not just skin, and hair pigment!
But for those that want to fully delve deeper into this, I would strongly encourage you to read the following:
King, R., M.D. (2001). “Melanin: A Key to Freedom”
www.amazon.com/Melanin-Key-Freedom-Richard-King/dp/1602810958/ref=sr_1_12?s=books&ie=UTF8&qid=1498928462&sr=1-12&keywords=melanin
Bynum, Bruce, E. (2014). “WHY DARKNESS MATTERS: (New and Improved): The Power of Melanin in the Brain”
https://www.amazon.com/WHY-DARKNESS-MATTERS-Improved-Melanin/dp/1502411172/ref=sr_1_5?s=books&ie=UTF8&qid=1498928462&sr=1-5&keywords=melanin
I plan to write much more about melanin in future posts, but suffice it to say we have been completely misinformed about:
- Its role as a source of mitochondrial energy,
- Its role as a super conductor of electricity – it is prevalent in key areas of the central nervous system (CNS).
- Its role as an iron-trapping agent, which is why its prevalence builds in lock step with the rising iron in the eyes.
- Its role as a master absorber of the complete visible light spectrum.
- Its role as a master absorber of sound in the inner ear.
- Its role as a critical facilitator of pineal health and function.
- Trust me, I could go on and on.
What is particularly poignant to understand is that melanin, in my humble opinion, has been hijacked and relegated to this lowly status as a pigment. It has also been methodically corralled into a master iron-trapping agent, which has completely diverted its ability to fulfil its majestic roles in the human body, as briefly and incompletely noted above.
In effect, melanin has become the “iron police force” responding to the tidal wave increase of iron in our bodies. Melanin is most effective at locking up that toxic heavy metal iron, and keeping it out of harm’s way. Here’s a clever twist, what’s a popular nickname for Policemen? Yes they’re called coppers! So we’ve got coppers putting this toxic metal behind bars!
How do you make melanin? The synthesis of melanin is entirely dependent on another key copper enzyme: Tyrosinase!
Where is that enzyme found in Mother Nature?
Why none other than wholefood vitamin-C that Albert Szent-Gyorgyi, PhD discovered in the 1930’s studying peppers from his hometown of Budapest, Hungary!
The most effective way to bind up melanin is with absurd levels of iron fortification in the food.
The most effective way to prevent melanin production is to drown the masses with ascorbic acid (devoid of any tyrosinase enzyme) and synthetic hormone-D that drives iron storage deeper and deeper into the tissue, which also causes renal potassium wasting (Ferris, 1962) thereby making the cells and the tissue more attractive to iron storage, and iron-induced oxidative stress!
Ferris, T.F., et al. (1962). “Renal potassium-wasting induced by vitamin D”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC291035/
The most effective way to distract or detract melanin entirely from the scene, and guarantee global neurodegeneration, is to do just what the system has done. As Jack Kruse, MD so aptly states, it’s been done right under our noses and we never even saw it!
Well, I’ll just speak for myself, I never saw this full court press of multiple iron factors to ensure neurodegeneration coming, despite my years of meticulous, minerals-based research. We can no longer ignore these conclusions.
So we learnt the saying is apt:
“Not known, because not looked for”
Okay, so what to do?
- Stop believing anything anyone has to say about neurodegeneration being a medical disease. (This is complete and utter fantasy on their part, and clearly a lack of awareness of the literature)
- Start to focus on understanding that excess, unbound iron, and the dysregulation that iron creates, is the very epicenter of this destructive metabolic dynamic.
- Stop thinking or believing that this is a genetic condition or a familial condition, that is simply not so, it is entirely an epigenetic one, or said another way, an environmental condition.
- Start understanding the scope and reach of iron-induced oxidative stress in all aspects of conditions and syndromes that you might be aware of or battling. They are truly all connected and riding on that “Ferrous Wheel” that runs your symptoms.
- Stop, believing that you are anemic! (Nothing could be farther from the truth)
- Please start the Root Cause Protocol to reverse this relentless pattern of mineral dysregulation that is triggered by iron.
I do apologize for the length of this particular post. It has been building for 6+ months, and I have wanted to share these important insights and discoveries for that entire time, but I also wanted to wait for just the right moment, when I felt more complete, and thus more confident, in my observations and conclusions.
This is our time! We absolutely need to pursue our health freedom, if for no other reason than to gain our medical independence from misguided clinical thinking that still believes neurodegeneration is a medical disease despite the countless articles that I’ve cited!
I trust that those of you who have labored and hung with me throughout this entire post would agree that that kind of clinical belief is seriously flawed, at best, and grossly irresponsible, at worst.
In subsequent posts and on-line chats, we will explore the important steps and options that we can all take to curb this iron influx of toxicity in our gut, and now as we’ve learned, our eyes.
A votre sante!
Morley M Robbins
For Facebook Discussion:
https://www.facebook.com/groups/MagnesiumAdvocacy/permalink/1400311870036878/