Iron Toxicity Post #47: The truth about the cause of mitochondrial dysfunction (as least as I see it)
This post is about the truth of the cause of Mitochondrial Dysfunction, at least as I see it.
Over the years, there have been certain phrases that have chaffed me, among the most offensive is this term: “Mitochondrial Dysfunction.”
It is a term that conjures up an equal amount of fear, wonderment and confusion like that often used phrase; “Oh, you have a lesion”
There are thousands and thousands of articles, both for the layperson and the scientist, written about this mitochondrial condition that is rapidly becoming a pandemic on this planet.
When all the dust settles, it’s not unlike writing a Magnus Opus about how to drive a car, with meticulous detail to every facet of that automotive experience with the notable exclusion of two key and fundamental dimensions to a successful drive:
- Forgetting to tell you that it’s really important to bring a key to start the engine
- Forgetting to tell you that the engine must have fuel to keep it running
Please take a good long look at the picture below.
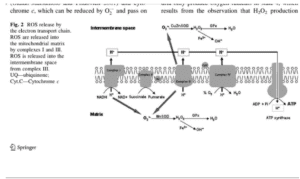
Figueiredo, P.A, et al. (2008). “The role of mitochondria in aging of skeletal muscle.”
I’ve been looking for this type of picture that dumbs down the mitochondrial electron transport chain (ETC) so that I can actually understand it! What I love is that this diagram helps to fill in many blanks that are ubiquitous and cleverly disguised in so much of the scientific literature.
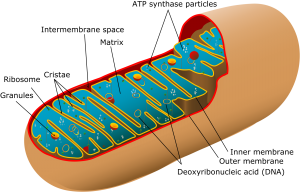
The mitochondria are where our adenosine tri-phosphate (ATP) gets made. ATP is the packet of energy that fuel our cellular, tissue, organ and bodily functions.
This takes place at Complex IV of the ETC. It’s ridiculously important. The mitochondrial cytochrome proteins that make up these complexes are the most extensively studied electron-transfer proteins.
The step that should make you sit up and take special notice is called cytochrome c oxidase (Complex IV) because it is here that four electrons get transferred to oxygen so that water (H2O) can be made so the ATP molecule can be re-made from ADP. As it turns out these cytochromes are actually heme proteins, which means that they contain ferrous (Fe2+) iron, which is the most reactive form of iron. This critical cytochrome c oxidase enzyme does not work without bioavailable copper, which is the key to make the ATP- engine work.
Who do you think is supplying the copper energy for this critical aspect of our metabolism? Ceruloplasmin! For those seeking mineral insight into this metabolic dynamic, please read Broderius, 2010:
Broderius, M., et al. (2010). “Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2854028/pdf/nihms-192848.pdf
Also note that there’s oxidative stress being given off on both sides of this mitochondrial process. Oxidative stress is a natural byproduct of these activities. It turns out the greatest source of endogenous oxidative stress occurs across these critical mitochondrial actions of Complex I through to V.
It is common to think that only copper and zinc superoxide dismutase (Cu/Zn-SOD) or known as SOD I, works the intracellular space (i.e. outside the mitochondria) and that only manganese superoxide dismutase (Mn-SOD) or known as SOD II, works the intra-mitochondrial membrane (i.e. inside the mitochondria). I used to think that was the case, too, until I found this ancient blockbuster (pdf.) article on ceruloplasmin (Goldstein, 1979):
Goldstein. I.M., et al. (1979). “Ceruloplasmin.”
https://www.jbc.org/article/S0021-9258(18)50692-X/pdf?fbclid=IwAR0S9X_gQrsWmZ-E4zW4cEfbYl6plv-LvT0IqlAx1AaSYYY0SA3airR3StI
What’s notable about the ceruloplasmin ferroxidase enzyme is that it is a recognized scavenger of superoxide radicals. They are those reactive oxygen (*O2-) molecules that are quite destructive. As you can see from the diagram above, they can and do devolve both inside and outside the mitochondria. They turn into the most destructive molecule in the body hydroxyl radical (OH-). This is the origin of what dings deoxyribonucleic acid (DNA) to create epigenetic chaos to support methylenetetrahydrofolate reductase (MTHFR).
This destructive molecule also leads to a breakdown in the efficiency and impact of the energy production of these mitochondria, which is known as chronic fatigue syndrome (CFS) as well a host of other chronic conditions of low energy.
You can now see how this destructive molecule also disrupts the cellular machinery of our body systems such as livers, hearts, brains, kidneys, joints, and muscles.
Now, can you all begin to see why this thing mitochondrial dysfunction is such a big deal, some cells have as many 200-500 mitochondria in them but I believe it is not nearly as complicated as it is methodically made out to be.
Furthermore, what I was not certain about until today is that ceruloplasmin is not just restricted to the cytoplasm (i.e. outside the mitochondria). It turns out that this scavenger is, for a fact, found inside the mitochondria, as well. It is well documented in these Russian researches!
Vasin, A.V. et al. (2005). “Mitochondrial ceruloplasmin of mammals.”
https://www.ncbi.nlm.nih.gov/m/pubmed/15773547/
Balevska, P., et al. (1975). “Studies on the transfer of copper from ceruloplasmin to mitochondria.”
https://www.ncbi.nlm.nih.gov/m/pubmed/1232838/
I have learned over the years to regard the abstracts that have no abstracts as being really important.
The plot begins to thicken with the benefit of these penetrating insights and here’s what I think is the blockbuster.
It turns out that 20% of the mitochondrial bi-lipid membrane is made up of a phospholipid called cardiolipin. While it is 20% of the total composition, it is actually 50% of the phospholipids found to be a part of the ETC (i.e. Complexes I – V) lipids. Here is the article that offers keen insight to the dynamics between cardiolipin and superoxide:
Soussi, B., et al. (1990). “H-n.m.r. evaluation of the ferricytochrome c-cardiolipin interaction: Effect of superoxide radiacals.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1136634/pdf/biochemj00192-0227.pdf
It turns out that cardiolipin is essential for the function of cytochrome c oxidase. Wow! I have read scores of articles on this Complex IV enzyme, cytochrome c oxidase, but I have never read that before. Their interaction is electrostatic in nature, as I’m sure everything is that happens in our bodies.
So what does this mean?
- Copper is the key to start this cytochrome c oxidase enzyme production of ATP
- Cardiolipin is the fuel upon which the cytochrome c oxidase enzyme works
What do the professionals from Dover have to say in the summary about the origin of mitochondrial dysfunction:
“Mitochondrial dysfunction… is related to a decreased cytochrome c oxidase activity, ultimately because of cardiolipin peroxidation [i.e. rusting of the fats] (Smith et al, 1980; Okayasu et al, 1985) causing a diminished cytochrome c binding to this enzyme.”
So in the absence or the presence of lowered production of ceruloplasmin, those ubiquitous superoxide (*O2-) molecules get further radicalized by ferrous (Fe2+) iron. Then into the OH- radical that then oxidizes the cardiolipin.
I happen to think it is relevant and most vital that ceruloplasmin brings both the copper (key) and ensures that the cardiolipin (fuel) does not get rusty as we talked about the car analogy earlier in the post.
Ceruloplasmin, as the Ferroxidase enzyme, converts ferrous (Fe2+) iron into ferric (3+) iron, the latter form of iron being what can then be used in cellular transport and other metabolic proteins to keep us healthy and energized. Isn’t it amazing that ceruloplasmin does this and more?
Even though this term mitochondrial dysfunction seems hopeless and it is a critical issue we all recognize and know. It can be whittled down to size and made far easier to comprehend and understand the why and how, and then we can prevent or reverse its development in our metabolism.
It is not a Medical Disease. Mitochondrial dysfunction is simply a clinical sign of Mineral Dysregulation – pure and simple.
Those who are seeking direction on what to do to address this, you can go here and get started: https://therootcauseprotocol.com
A votre sante!
MORLEY M. ROBBINS
For Facebook Discussion:
www.facebook.com/groups/MagnesiumAdvocacy/permalink/1209061335828600/